Instituto de Investigação e Inovação em Saúde, Universidade do Porto
Rua Alfredo Allen, 208 4200-135 Porto, Portugal
Phone: +351 226 074 900
Email: info@i3s.up.pt
The research of our group is clustered in two main lines:
Inflammatory Bowel Disease (IBD) is a chronic disorder that affects the gastrointestinal tract. Despite recent advancements in clinical management, IBD continues to be one of the primary contributors to morbidity. In this sense, tackling the initial steps of inflammation may bring novel insights to early diagnosis and improve the prediction and prevention of IBD.
The goal of this project is to address whether and how the modifications of host cell glycosylation impact the intestinal environment, in particular innate and adaptive immune responses, in order to pinpoint the cellular and molecular mechanisms governing the host glycome-microbial network associated with the initiation of intestinal inflammation.
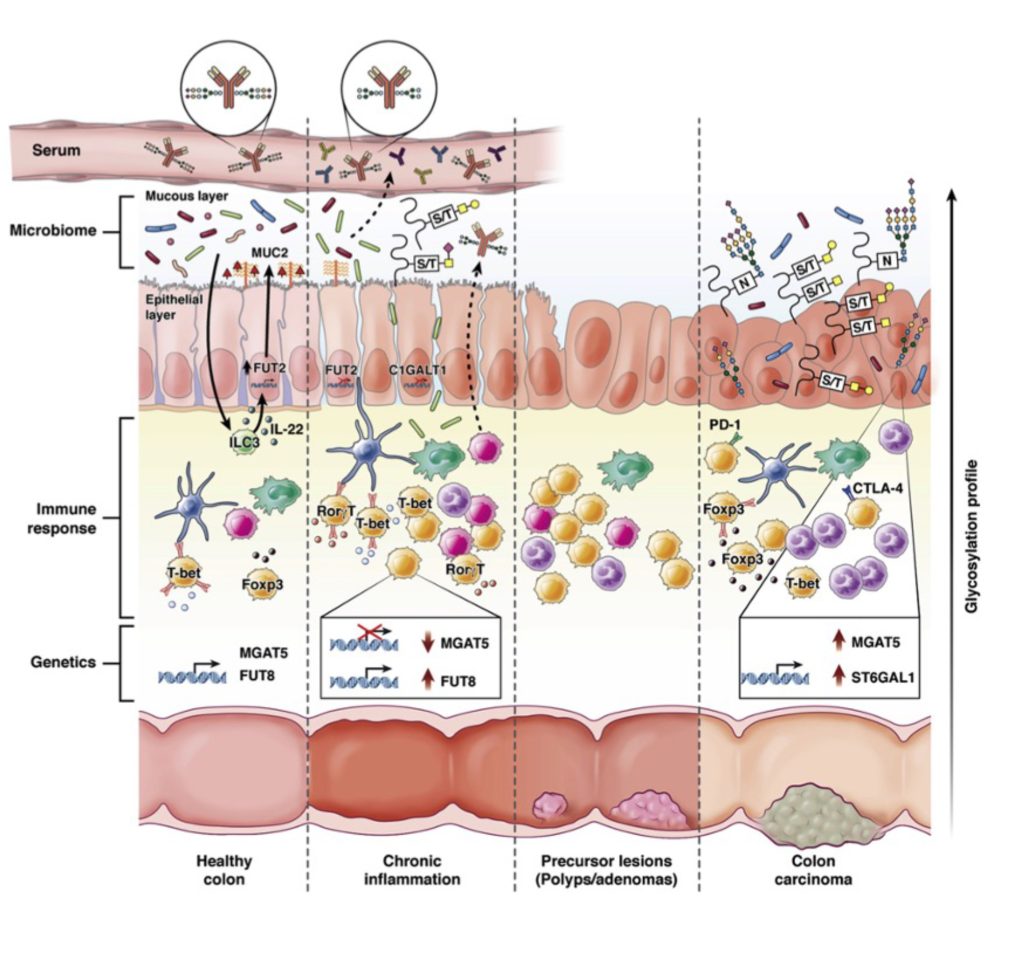
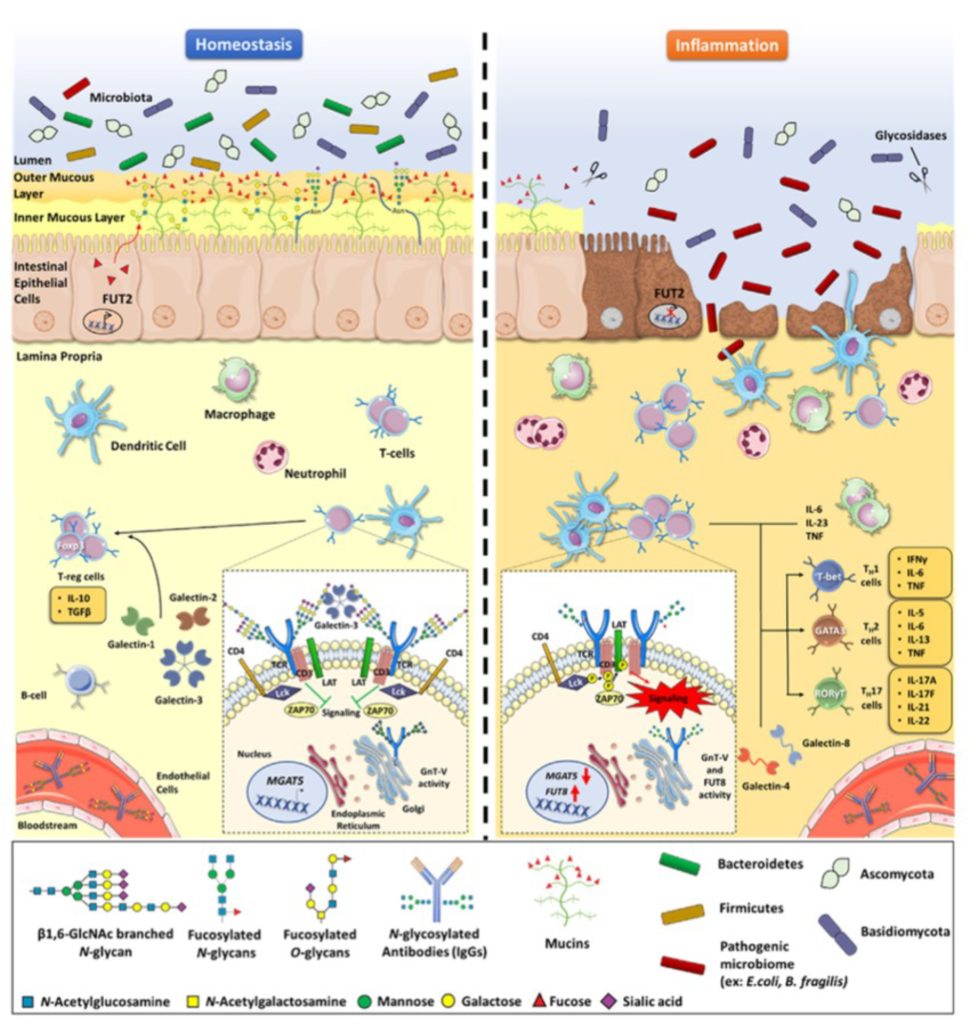
The human gut houses a diverse community of microorganisms, the microbiome. The perturbation of the symbiotic relationship between microbes and the intestinal immune system is shown to contribute to the onset of many inflammatory diseases, such as IBD. Host glycocalyx (repertoire of glycans/sugar chains at the surface of gut mucosa) is a major biological and physical interface between intestinal mucosa and microorganisms and should be an important modulator of host-microbe homeostasis. In this project, we aim to investigate how the host glycocalyx shapes the composition and function of gut microbiota associated with inflammation initiation and IBD development.
Glycans are molecules that allow the immune system to differentiate between self and non-self. However, changes in the glycosylation profile occur in the transition from normal to disease conditions. In this project, we are exploring the role of glycans in the loss of self tolerance by studying the impact on Systemic Lupus Erythematosus (SLE) immunopathogenesis. Recently, we characterized the glycan signature of SLE patients, where an accumulation of less complex N-glycans (mannose-enriched N-glycans) was observed. This abnormal glycosylation seems to be associated with an infiltration of pathogenic γδT cells that contribute to the establishment of pro-inflammatory pathways. The main goal of this project is to investigate the impact of an altered N-glycosylation pathway as a new mechanism associated with the breach of immunotolerance and the rise of autoimmunity, envisioning novel diagnosis and prognosis markers as well as new therapeutic targets for autoimmune diseases
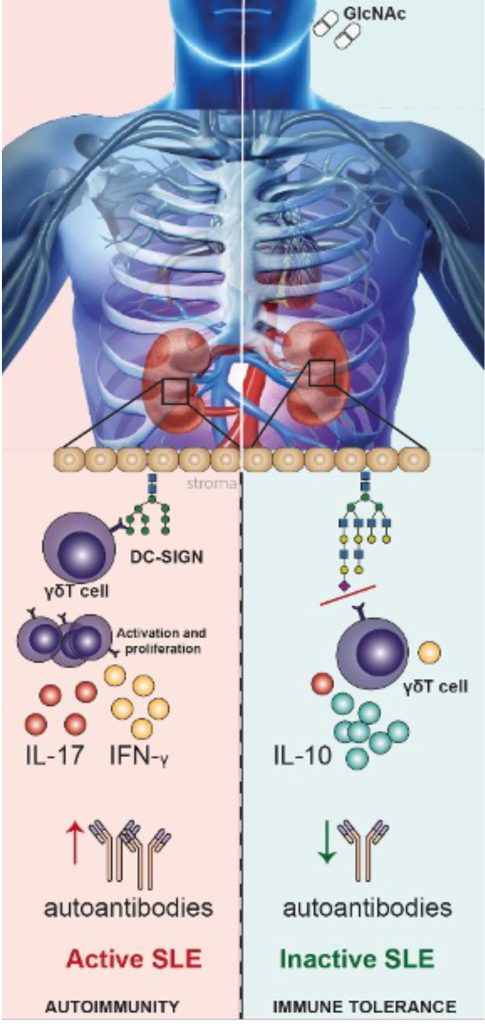

Immune-mediated diseases (IMIDs) are a group of chronic inflammatory conditions characterized by a hyperimmune response in a susceptible host. IMIDs are debilitating and life-threatening diseases, Rheumatoid Arthritis, Systemic Lupus Erythematosus, juvenile diabetes (type 1), and Inflammatory Bowel Disease among others. The main goal of this project is to understand when, why, and how glycans modifications act as a master switch that triggers abnormal immune cell activity, thus resulting in the production of pathogenic autoantibodies associated with the loss of immune tolerance and the development of autoimmunity. Ultimately, we want to interfere/modify this master switch and thereby exploit/ analyse whether this constitutes an opportunity to prevent IMIDs in high-risk individuals.
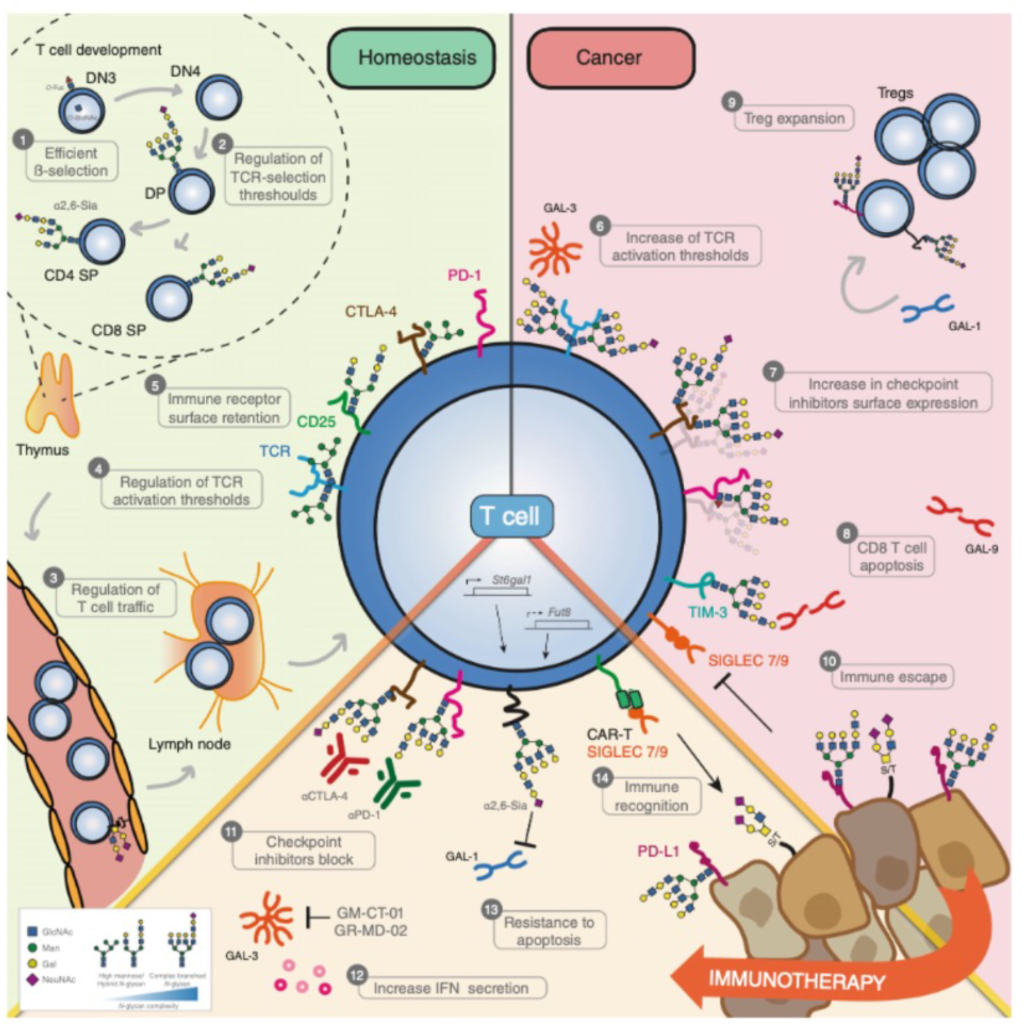
Changes in cellular glycosylation signature are a hallmark of malignant transformation. In recent years, we have been dedicating our research to the field of cancer glycobiology. We discovered that the abnormal overexpression of branched N-glycans by malignant cells constitutes a novel immune checkpoint in cancer by regulating both innate and adaptive immune responses in colorectal cancer (CRC). However, whether and how this abnormal cellular glyco profile occurs in pre-malignancy remains completely unknown. Moreover, whether this early glycoimmunology remodeling acts as a switching event toward malignant transformation has never been addressed. The main goal of this project is to explore novel mechanisms of tumor immune evasion, based on the abnormal expression of glycans by premalignant colonic cells and infiltrating T cells, either in sporadic, hereditary, or inflammatory bowel disease-associated CRC. This pioneering project tackles a key challenge in cancer prevention, leading to the improvement of diagnostic tools and immunopreventive strategies, aiming to impact globally the oncology field.